Right Drugs
At Onco3R, we take a modality-agnostic strategy to unlock the full therapeutic potential of clinically validated targets. Our pipeline includes both small molecules and antibody-based therapies, each tailored to address the complex disease biology in oncology and immunology
Our Pipelines
A portfolio of 5 programs in immunology & oncology
Unmet need in autoimmune diseases
At Onco3R, we are building on 15 years of experience in the discovery and development of SIK family inhibitors to treat autoimmune diseases. We have developed SIK3 inhibitors that we believe have the potential to revolutionise the way autoimmune diseases are treated.
SIKs, or salt-induced kinases, are a family of proteins that play roles in a wide array of biological processes, including inflammation. Three different isoforms exist, SIK1, SIK2 and SIK3. We have identified that SIK3 plays a key role in regulating pathogenic processes involved in autoimmune diseases and that by inhibiting it, the pathogenic processes and the progression of disease can be inhibited.
SIK3 inhibitor O3R-5671
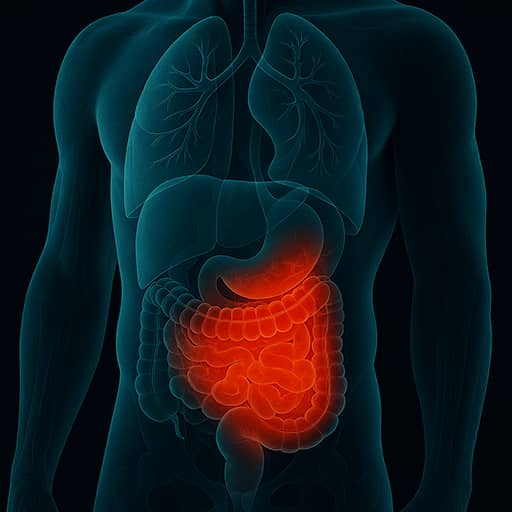
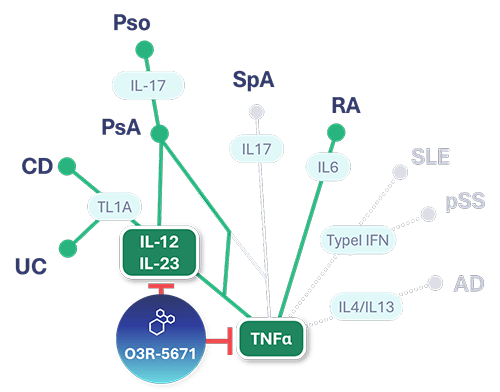
Our lead asset, O3R-5671, is a highly potent and specific SIK3 inhibitor that has been shown to target a variety of cytokines that cause disease in a variety of autoimmune diseases including ulcerative colitis (UC), Crohn’s disease (CD), psoriasis (Pso), psoriatic arthritis (PsA) and rheumatoid arthritis (RA).
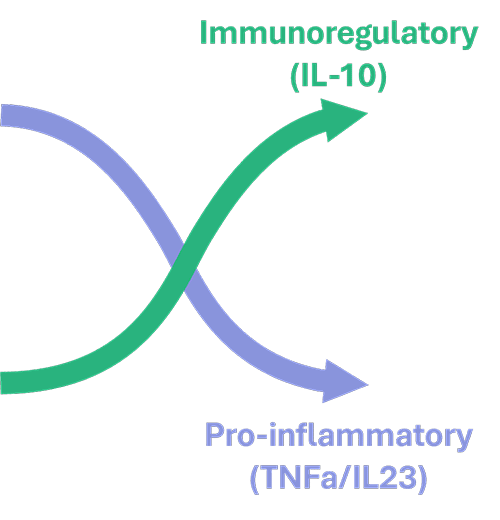
O3R-5671 targets the SIK3 enzyme which in turn impacts other proteins resulting in reduced transcription of genes involved in inflammation including tumor-necrosis factor alpha (TNFa) and interleukin 23 (IL-23) and increased transcription of immunoregulatory genes such as IL-10. Numerous studies have been conducted in translational models of autoimmune diseases which indicate that O3R-5671 is able reduce the severity of these diseases. We have also conducted extensive toxicology studies and are now ready to investigate O3R-5671 in humans.
Clinical study
A clinical study of O3R-5671 which will investigate various doses administered as single and multiple doses is ongoing. This study will establish the safety and pharmacokinetics of O3R-5671 and provide insights into how it is able to dampen the immune response in the blood of people who have received it.
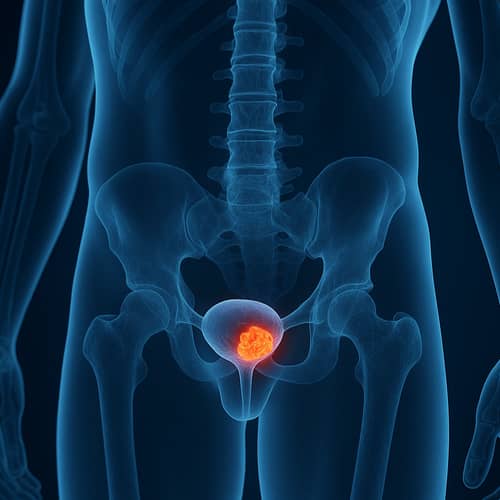
Targeting bladder cancers addicted to oncogenic FGFR3
FGFR3 mutations and fusions are oncogenic drivers in ~50% of bladder cancers and are associated with poor survival in metastatic settings. A first-generation pan-FGFR inhibitor (FGFR1-4) has been approved for second-line treatment of advanced or metastatic urothelial carcinoma with genetic FGFR3 alterations. However, resistance mutations and dose-limiting toxicities from off-target inhibition of FGFR1/2/4 limit its clinical potential, preventing its use in earlier treatment lines or disease stages. There is therefore a clear need for a selective FGFR3 small-molecule inhibitor that is active against resistance mutations to fully unlock the potential of FGFR3 inhibition in bladder cancer.
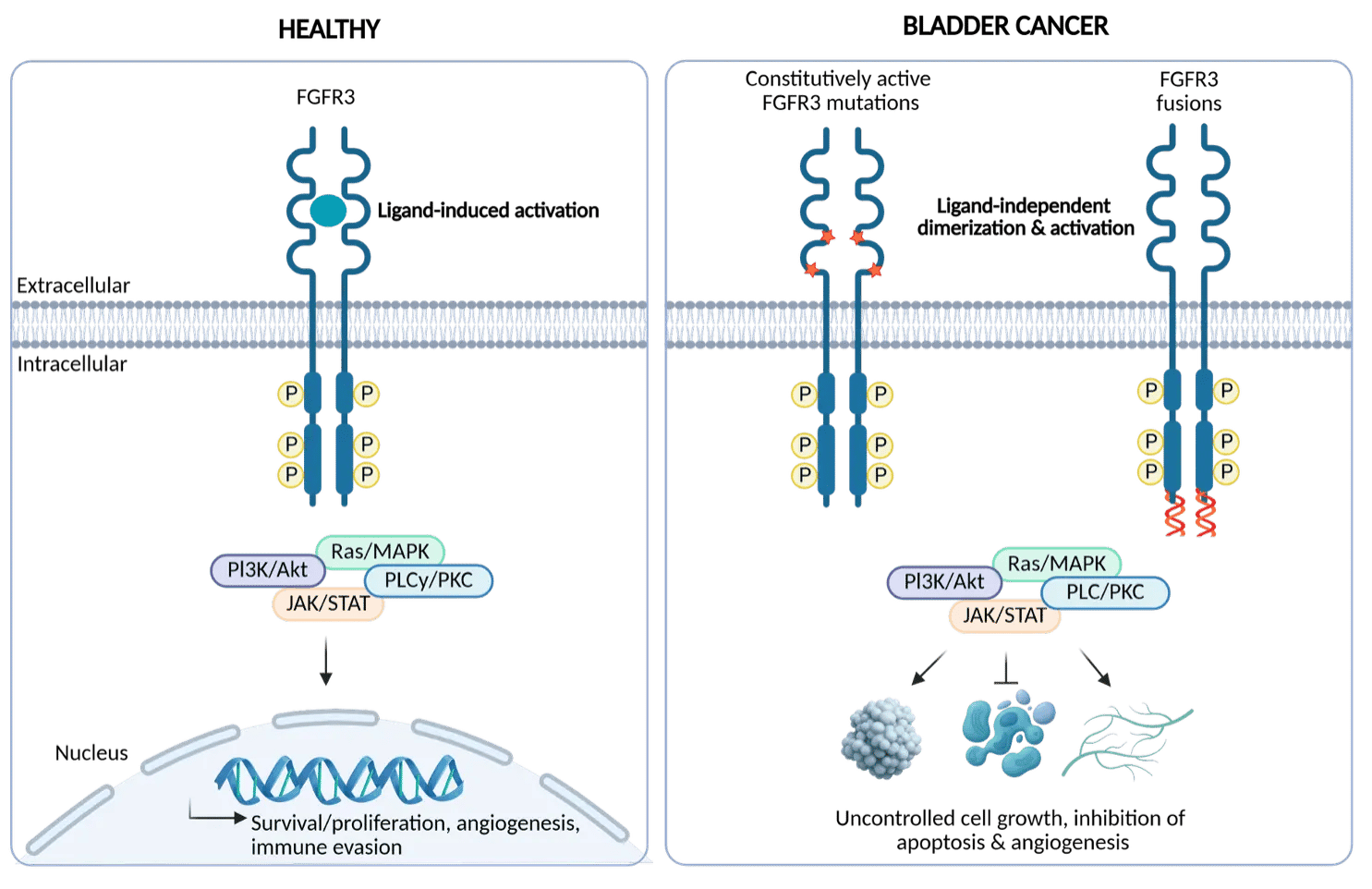
Onco3R FGFR3 selective small molecule
Our patient-centric approach, integrating unique translational modelling with rational, structure-based, and AI-augmented drug design, has led to the identification of a proprietary FGFR3-selective inhibitor series with best-in-class potency and safety. These leads are currently being profiled as potential clinical candidates.
Targeting SMARCA2 in SMARCA4 deficient non-small cell lung cancer
Loss of SMARCA4 occurs in ~5% of NSCLC cases, defining a distinct patient population with poor prognosis and a 5-year survival rate below 10%. While SMARCA2 inhibition has long been established to induce synthetic lethality in SMARCA4-deficient cells, translation into the clinic has been hampered by lack of selectivity against SMARCA4.
First-generation non-selective SMARCA2/4 inhibitors were discontinued due to limited target coverage and toxicities linked to SMARCA4 inhibition. Thus, there is a clear need for an oral, potent, and selective SMARCA2 small-molecule inhibitor to fully realize the therapeutic potential of this target.
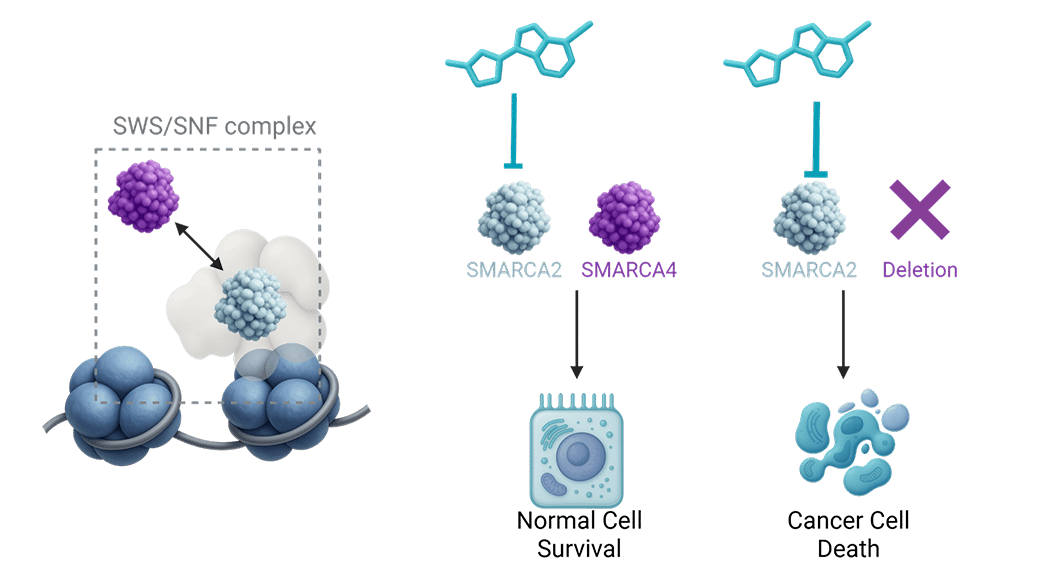
Onco3R SMARCA2 selective small molecule inhibitor
Our patient-centric approach, integrating deep translational science with rational, structure-based, and AI-augmented drug design, has yielded a proprietary SMARCA2-selective inhibitor series with best-in-class potency and safety. These leads are currently being profiled as potential clinical candidates.
Targeting cancers addicted to the oncogenic P53 Y220C mutation
The Y220C mutation in the p53 protein occurs in ~1% of all cancers, across more than 30 tumor types, including non-small cell lung cancer (NSCLC). This mutation induces p53 misfolding and inactivation, but also creates a unique, druggable pocket on the protein surface, making it an attractive therapeutic target.
First-generation small-molecule reactivators, designed to bind this pocket and restore p53 tumor-suppressive function, have shown clinical efficacy in patients harboring Y220C mutations. However, their modest potency requires high dosing, which increases the risk of adverse events and narrows the therapeutic window, both as monotherapy and in combination regimens. This highlights the need for a highly potent p53 Y220C small-molecule reactivator to fully unlock the therapeutic potential of this target.
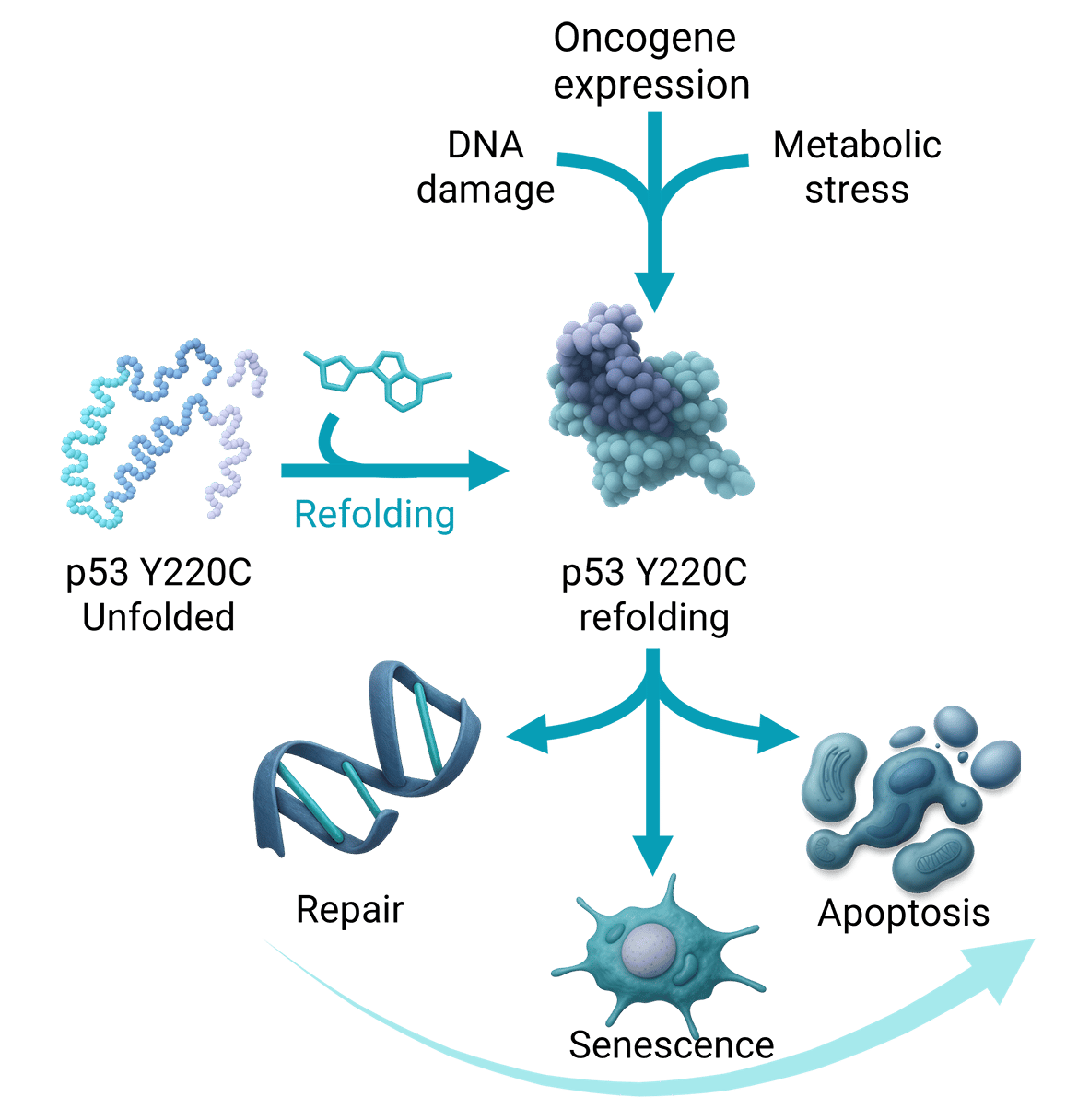
Onco3R P53 Y220C selective small molecule reactivator
Our patient-centric approach, leveraging clinical data from first-generation p53 reactivators, combined with rational, structure-based, and AI-augmented drug design, has led to the development of a proprietary p53 Y220C selective reactivator series with best-in-class potency. These leads are currently being profiled as potential clinical candidates.
Our Programs
Our work is concentrated on two key therapeutic areas where we can make the greatest impact for patients.
Oncology
In Oncology, we develop precision oncology therapies, centered on clinically validated cancer targets and aim to overcome limitations of first-generation therapies such as low target coverage, off-target toxicity, and resistance.
Explore Oncology PipelineImmunology
In Immunology, we target autoimmune diseases with a novel mechanism of action by inhibiting the SIK3 kinase, resulting in modulation of both immunoregulatory and anti-inflammatory pathways.
Explore Immunology PipelineOur Focus
Unlocking the full potential of therapeutic targets with best-in-class medicines
Optimal target/pathway coverage
Improving the efficacy of treatments by addressing low target coverage
High selectivity
Reducing adverse reactions by ensuring high selectivity and minimizing off-target effects
Minimal resistance
Preventing the lack or loss of response by minimizing treatment resistance