Right Strategy
In autoimmune diseases and oncology, a significant proportion of patients either fail to respond to approved therapies or suffer from significant side effects. At Onco3R, we are committed to developing precision medicines that maximize therapeutic benefit while enhancing safety and convenience for patients. We focus on clinically validated targets and select the right therapeutic modalities, whether small or large molecules, to provide the right drugs to the right patients.
Our Focus
Unlocking the full potential of therapeutic targets with best-in-class medicines
Optimal target/pathway coverage
Improving the efficacy of treatments by addressing low target coverage
High selectivity
Reducing adverse reactions by ensuring high selectivity and minimizing off-target effects
Minimal resistance
Preventing the lack or loss of response by minimizing treatment resistance
Our Science
Unique proprietary translational modelling
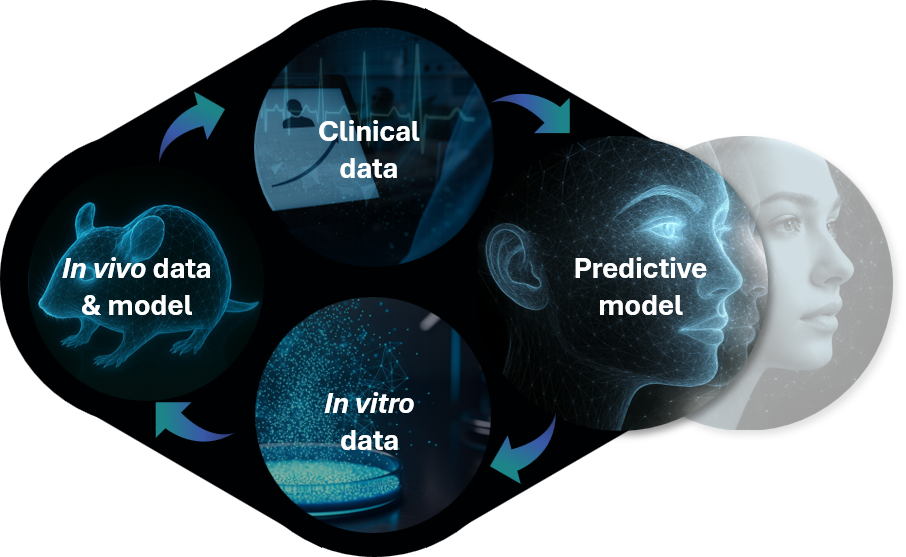
We are patient-centric and committed to addressing the unmet needs left by first-generation therapies. Our strategy is built on a robust translational framework that allows us to identify and overcome efficacy and safety gaps in current treatments, unlocking the full therapeutic potential of our targets.
Our proprietary translational modelling uniquely integrates clinical data with in vitro and in vivo translational insights to analyze the limitations of currently approved therapies and guide the design of best-in-class drugs.
Data driven drug discovery
We integrate advanced computational tools and structure-based drug design to drive medicinal chemistry and antibody engineering, accelerating the development of best-in-class targeted therapies. By combining AI-driven predictions with high-resolution protein structures, we optimize small-molecule binding and antibody interactions with their cognate target.
This approach forms the foundation of the Design–Predict–Make–Test–Analyze cycle, enabling faster and more informed decision-making. Our platform continuously learns from experimental results and provides real-time feedback to scientists, empowering them to focus on what they do best: designing and delivering the next generation of best-in-class treatments with greater efficiency and reduced risk.
Early derisking and rapid POC
By integrating early pharmacokinetic and safety assessments, we proactively reduce the risk of late-stage attrition and accelerate the path to viable treatments.
Our combined expertise in clinical development, pharmacology, operations, and regulatory affairs enables us to rapidly generate proof-of-concept data—validating our innovations in real-world settings.