Precision Oncology
Developing next-generation cancer therapies targeting solid tumors and hematologic malignancies through novel mechanisms of action and precision medicine approaches.
FGFR3 Program
Targeting bladder cancers addicted to oncogenic FGFR3
FGFR3 mutations and fusions are oncogenic drivers in ~50% of bladder cancers and are associated with poor survival in metastatic settings. A first-generation pan-FGFR inhibitor (FGFR1-4) has been approved for second-line treatment of advanced or metastatic urothelial carcinoma with genetic FGFR3 alterations.
However, resistance mutations and dose-limiting toxicities from off-target inhibition of FGFR1/2/4 limit its clinical potential. There is therefore a clear need for a selective FGFR3 small-molecule inhibitor that is active against resistance mutations to fully unlock the potential of FGFR3 inhibition.
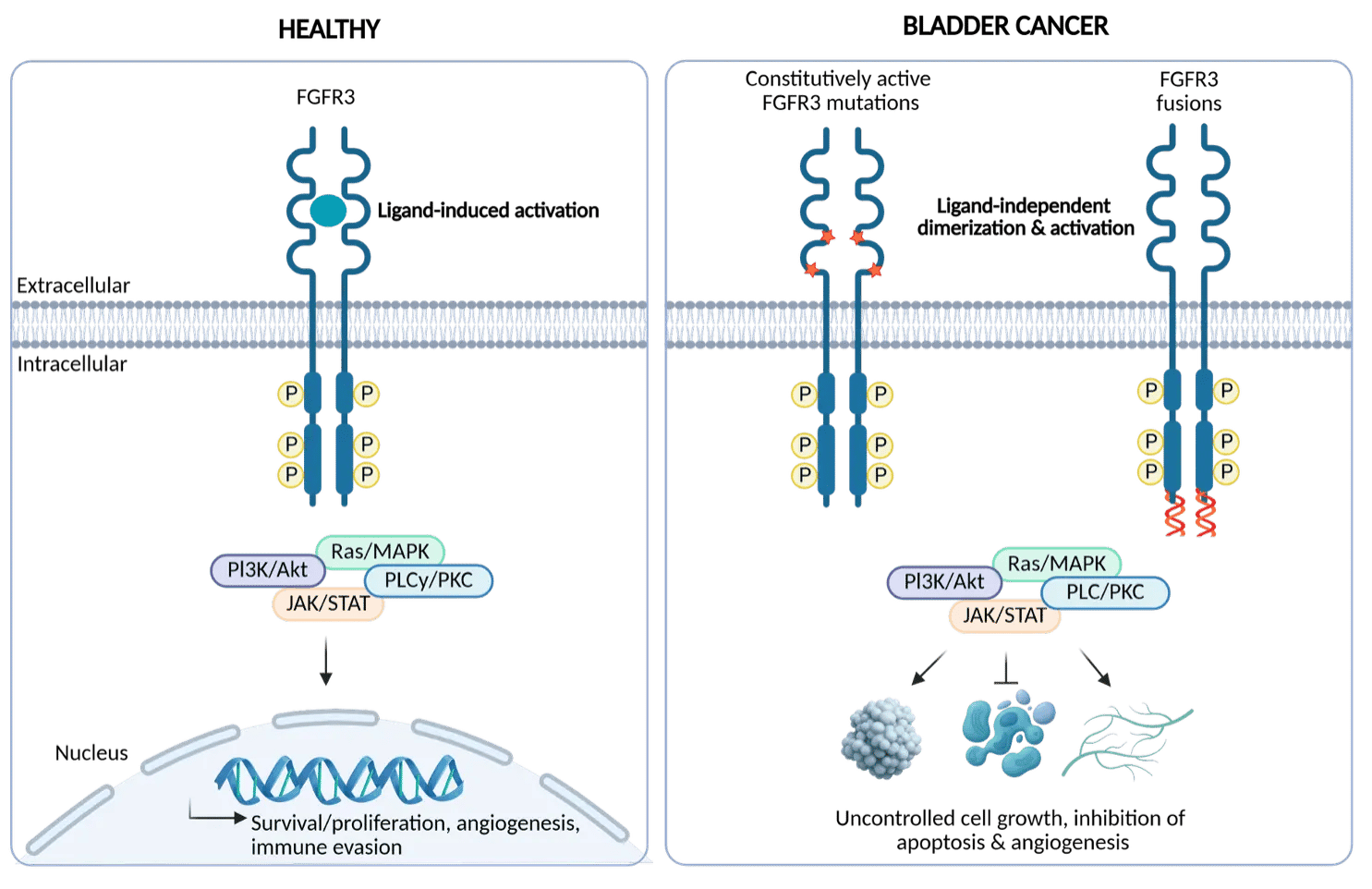
Onco3R FGFR3 selective small molecule
Our patient-centric approach, integrating unique translational modelling with rational, structure-based, and AI-augmented drug design, has led to the identification of a proprietary FGFR3-selective inhibitor series with best-in-class potency and safety. These leads are currently being profiled as potential clinical candidates.
SMARCA2 Program
Targeting SMARCA2 in SMARCA4 deficient non-small cell lung cancer
Loss of SMARCA4 occurs in ~5% of NSCLC cases, defining a distinct patient population with poor prognosis and a 5-year survival rate below 10%. While SMARCA2 inhibition has long been established to induce synthetic lethality in SMARCA4-deficient cells, translation into the clinic has been hampered by lack of selectivity against SMARCA4.
First-generation non-selective SMARCA2/4 inhibitors were discontinued due to limited target coverage and toxicities linked to SMARCA4 inhibition. Thus, there is a clear need for an oral, potent, and selective SMARCA2 small-molecule inhibitor.
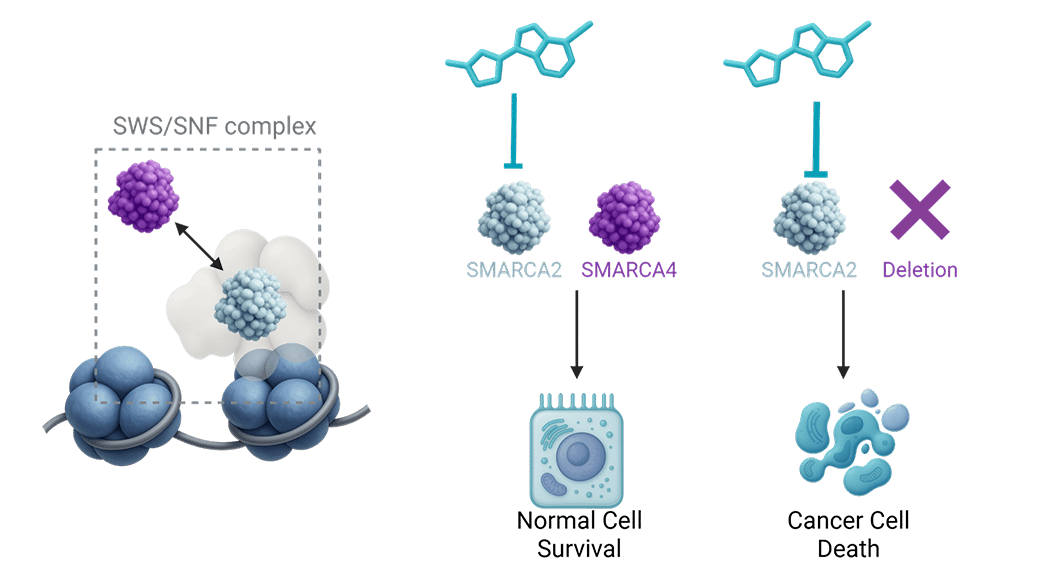
Onco3R SMARCA2 selective small molecule inhibitor
Our patient-centric approach, integrating deep translational science with rational, structure-based, and AI-augmented drug design, has yielded a proprietary SMARCA2-selective inhibitor series with best-in-class potency and safety. These leads are currently being profiled as potential clinical candidates.
P53 Program
Targeting cancers addicted to the oncogenic P53 Y220C mutation
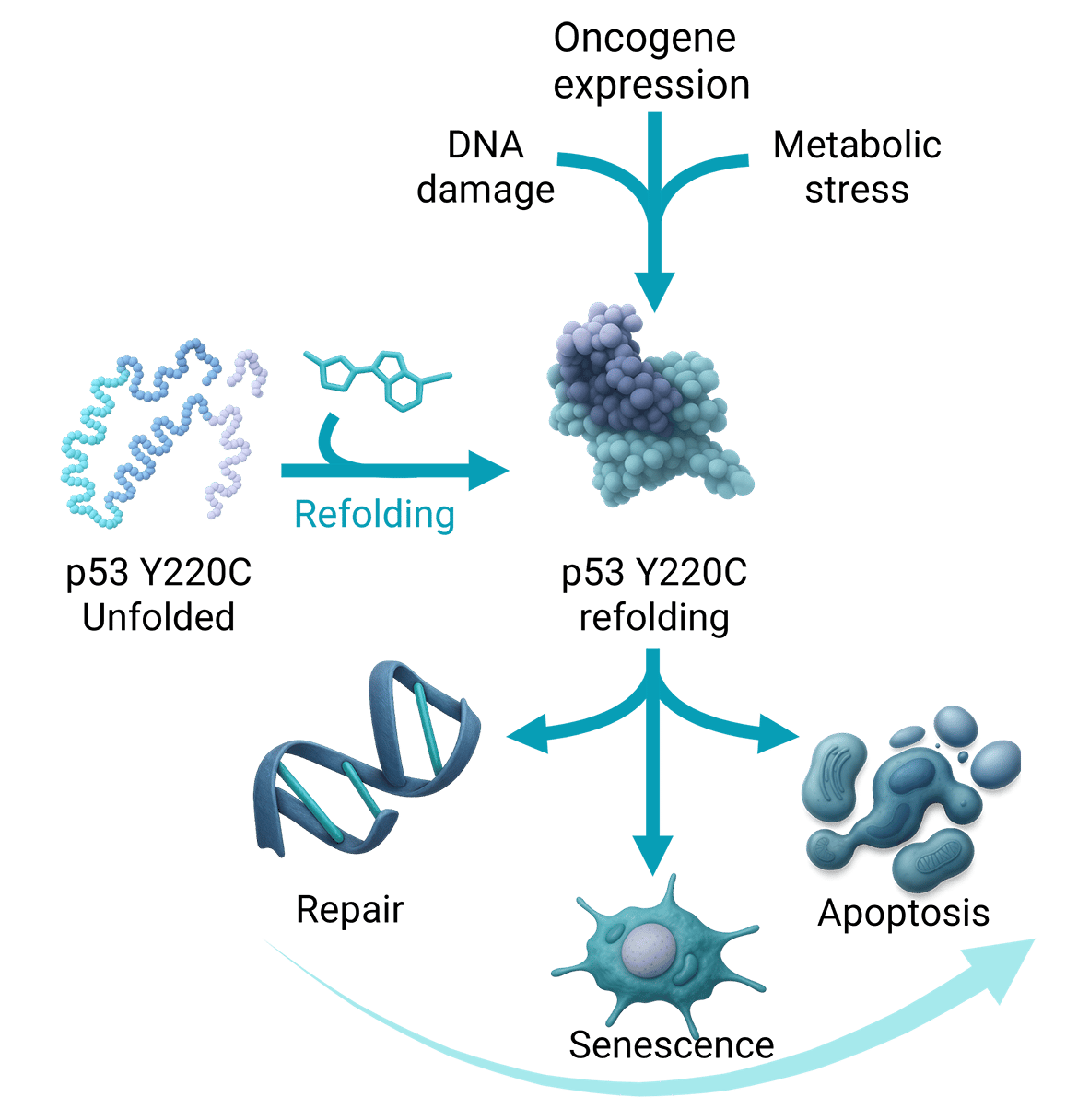
The Y220C mutation in the p53 protein occurs in ~1% of all cancers, across more than 30 tumor types, including non-small cell lung cancer (NSCLC). This mutation induces p53 misfolding and inactivation, but also creates a unique, druggable pocket on the protein surface.
First-generation small-molecule reactivators have shown clinical efficacy but often require high dosing due to modest potency. This highlights the need for a highly potent p53 Y220C small-molecule reactivator to fully unlock the therapeutic potential of this target.
Onco3R P53 Y220C selective small molecule reactivator
Our patient-centric approach, leveraging clinical data from first-generation p53 reactivators, combined with rational, structure-based, and AI-augmented drug design, has led to the development of a proprietary p53 Y220C selective reactivator series with best-in-class potency. These leads are currently being profiled as potential clinical candidates.